Research
Our research spans multiple areas of modern biology, from molecular mechanisms to systems-level understanding. We employ cutting-edge experimental and computational approaches to address fundamental questions in life sciences.
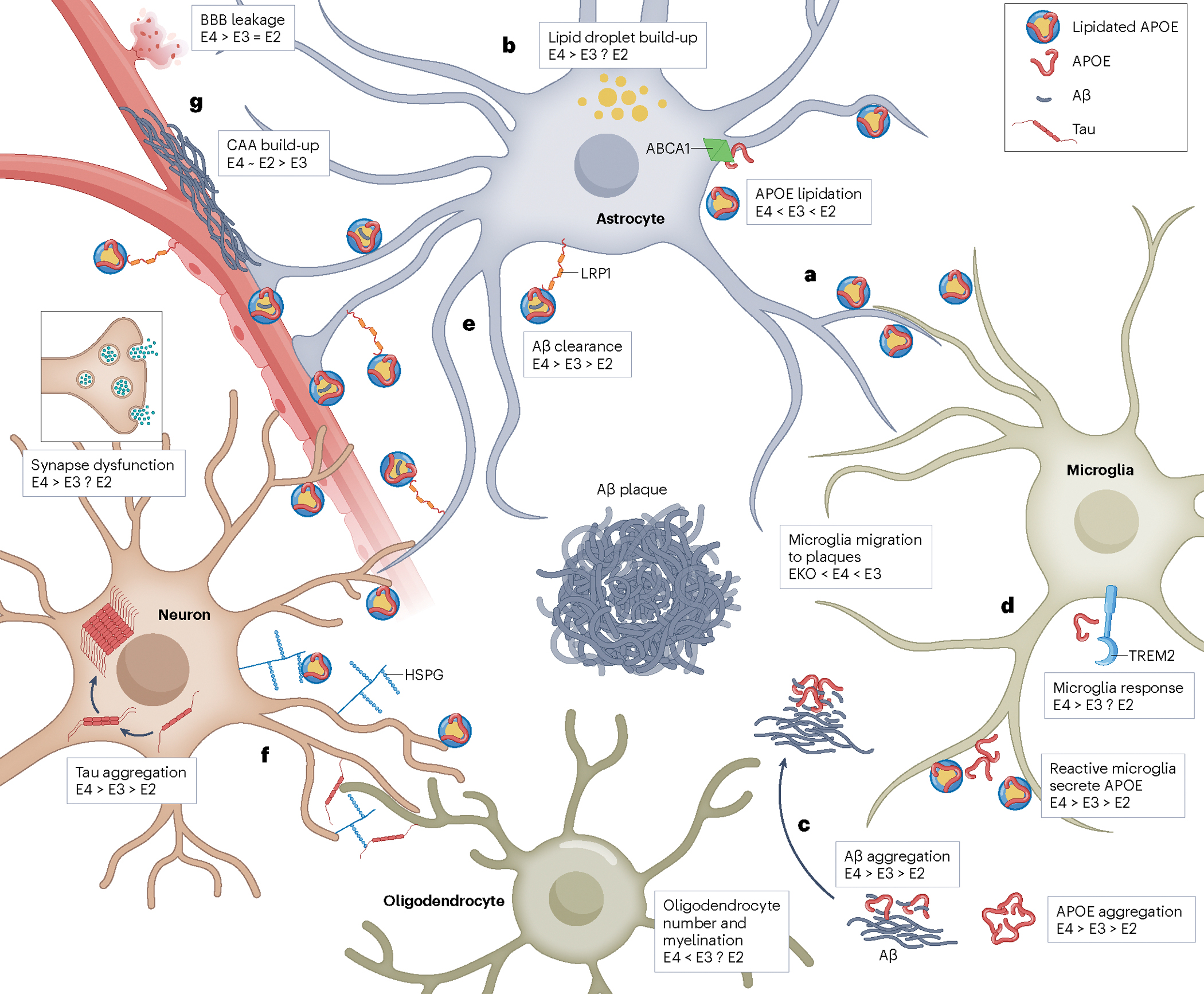
APOE and Neurodegenerative Diseases
ActiveProject Overview
Apolipoprotein E (APOE), a lipid transport protein primarily expressed by astrocytes and microglia in the central nervous system, plays a multifaceted role in maintaining blood-brain barrier (BBB) integrity and regulating neuroinflammation. Recent work highlights how isoform-specific effects of APOE, particularly the pathogenic APOE4 variant, contribute to BBB dysfunction, increased neurodegeneration, and cognitive decline. We investigate the mechanistic underpinnings by which APOE4 disrupts astrocyte-endothelial signaling, leading to BBB permeability (Jackson et al., Brain, 2022), and how APOE-driven pathology exacerbates Alzheimer’s disease (AD) progression (Jackson et al., Nat Rev Neurol, 2024). In parallel, we explore therapeutic strategies such as APOE2 gene therapy, which has been shown to reduce amyloid burden and neuroinflammation in AD mouse models (Jackson et al., Mol Ther, 2024). Together, our research aims to define the central and peripheral roles of APOE in neurovascular dysfunction and neurodegeneration.
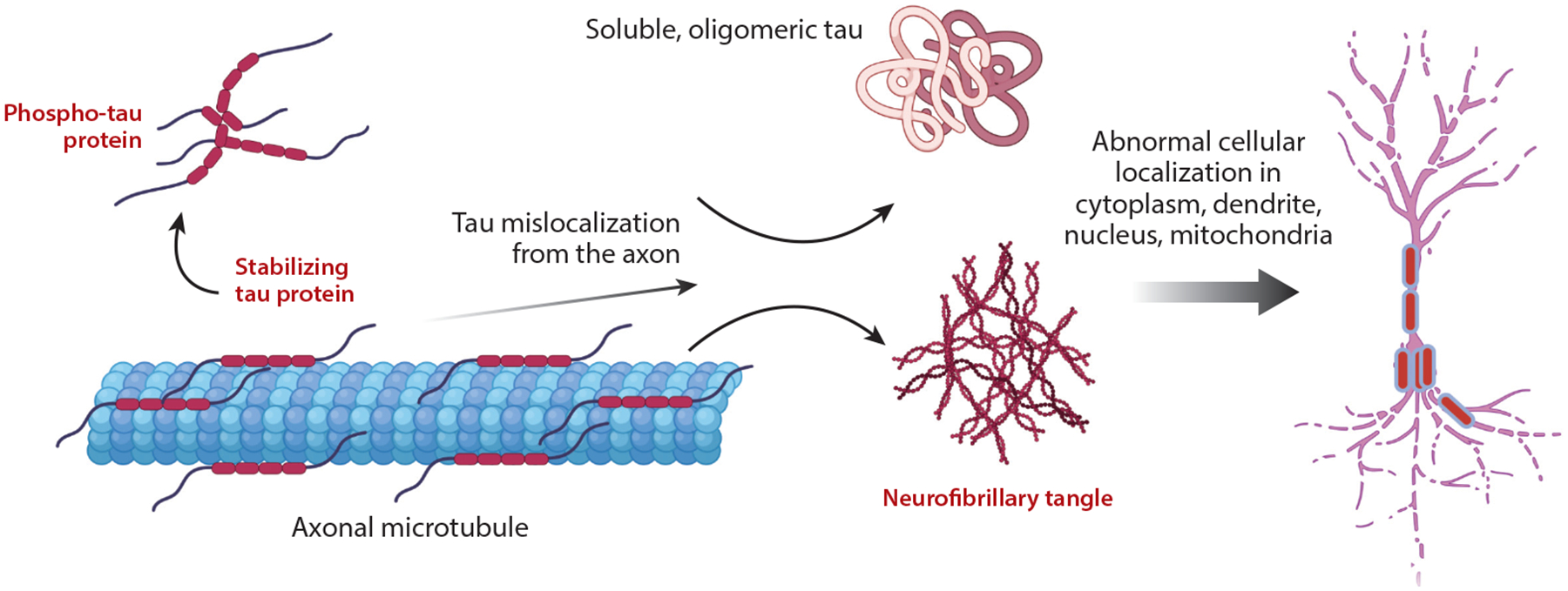
Tau Pathology and Mechanisms
ActiveProject Overview
Our lab focuses on understanding tau kinetics, including fibrillar and oligomeric forms, to elucidate their replication and spreading rates in the brain, key to Alzheimer’s progression. Using chemical kinetics, proteomics, and machine learning, we quantify tau aggregation and investigate why therapies effective in mouse models often fail in humans. We study tau-related neuronal resilience, GWAS-validated tauopathy genes (e.g., JADE1), and tau’s impact on neuronal firing critical for memory. We also examine pathogenic tau’s role in activating transposable elements and mislocalization of nuclear pore proteins like NUP98.
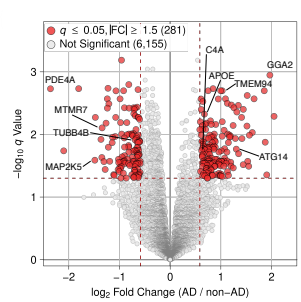
Transcriptomics and Neurodegeneration
ActiveProject Overview
We investigate the genomic and transcriptomic alterations underlying neurodegenerative diseases. Our findings reveal that neuronal DNA damage accumulates progressively in Alzheimer’s disease, driven by pathogenic mechanisms. We analyze region-specific endothelial cell heterogeneity and transcriptomic responses to amyloid-β plaques, tau tangles, and APOE variants. Additionally, we explore astrocyte transcriptomic changes over disease progression and identify therapeutic targets such as STMN2 and UNC13A in TDP-43 proteinopathies.
Publications
- Das, S. et al. (2023). “Distinct transcriptomic responses to Aβ plaques, neurofibrillary tangles, and APOE in Alzheimer’s disease.” Alzheimer’s & Dementia 20(1), 74-90.
- Wachter, A. et al. (2024). “Landscape of brain myeloid cell transcriptome along the spatiotemporal progression of Alzheimer’s disease reveals distinct sequential responses to Aβ and tau.” Acta Neuropathologica 147(1), 65.
- Collins, M. et al. (2025). “Ubiquitin-Proteasome System Dysregulation in Alzheimer’s Disease Impacts Protein Abundance.” [Preprint]