Hyman Lab
A collaborative research laboratory with a focus on understanding Alzheimer's etiology

Research Areas
APOE and Neurodegenerative Diseases
Project Overview
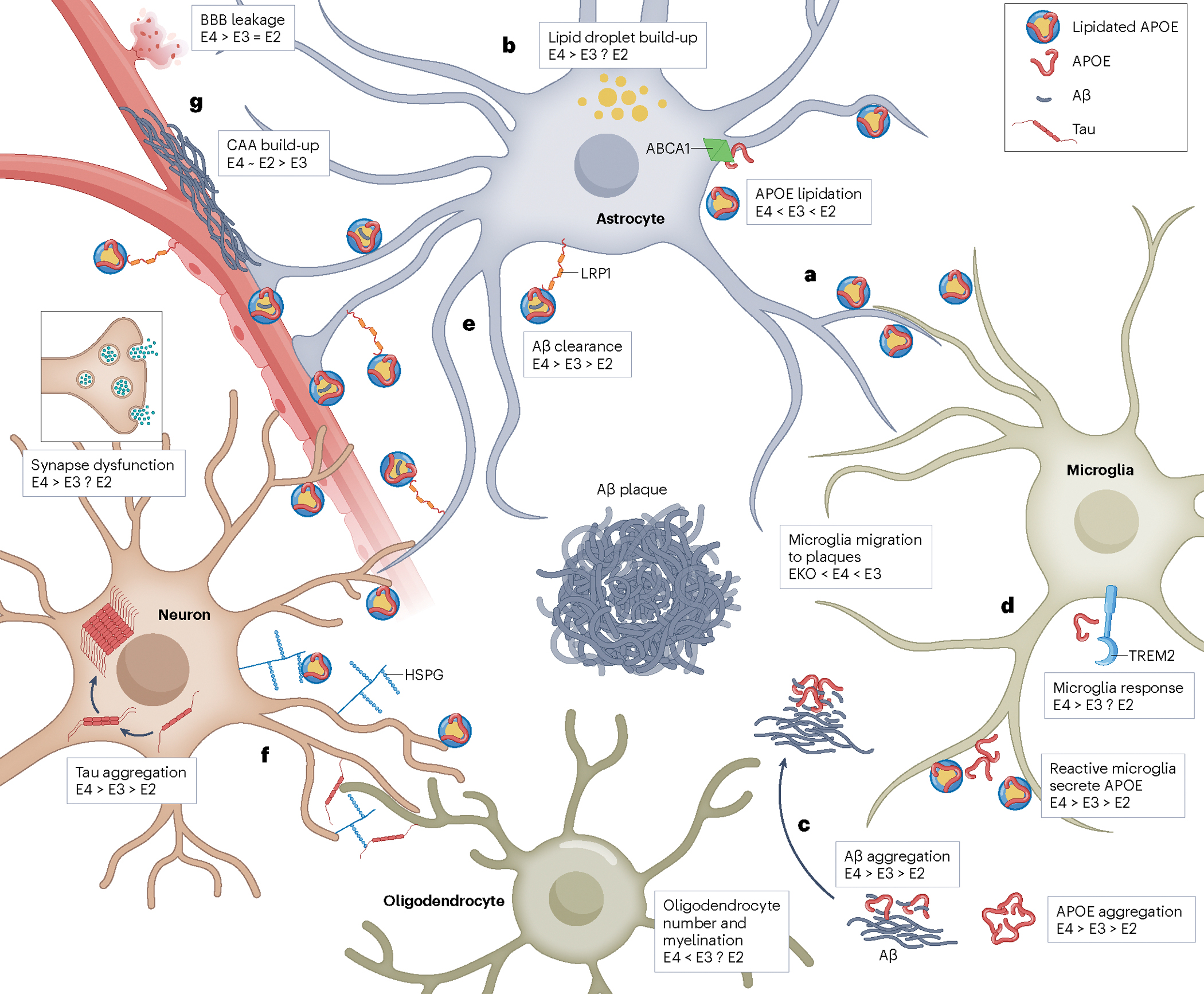
Apolipoprotein E (APOE), a lipid transport protein primarily expressed by astrocytes and microglia in the central nervous system, plays a multifaceted role in maintaining blood-brain barrier (BBB) integrity and regulating neuroinflammation. Recent work highlights how isoform-specific effects of APOE, particularly the pathogenic APOE4 variant, contribute to BBB dysfunction, increased neurodegeneration, and cognitive decline. We investigate the mechanistic underpinnings by which APOE4 disrupts astrocyte-endothelial signaling, leading to BBB permeability (Jackson et al., Brain, 2022), and how APOE-driven pathology exacerbates Alzheimer’s disease (AD) progression (Jackson et al., Nat Rev Neurol, 2024). In parallel, we explore therapeutic strategies such as APOE2 gene therapy, which has been shown to reduce amyloid burden and neuroinflammation in AD mouse models (Jackson et al., Mol Ther, 2024). Together, our research aims to define the central and peripheral roles of APOE in neurovascular dysfunction and neurodegeneration.
Learn More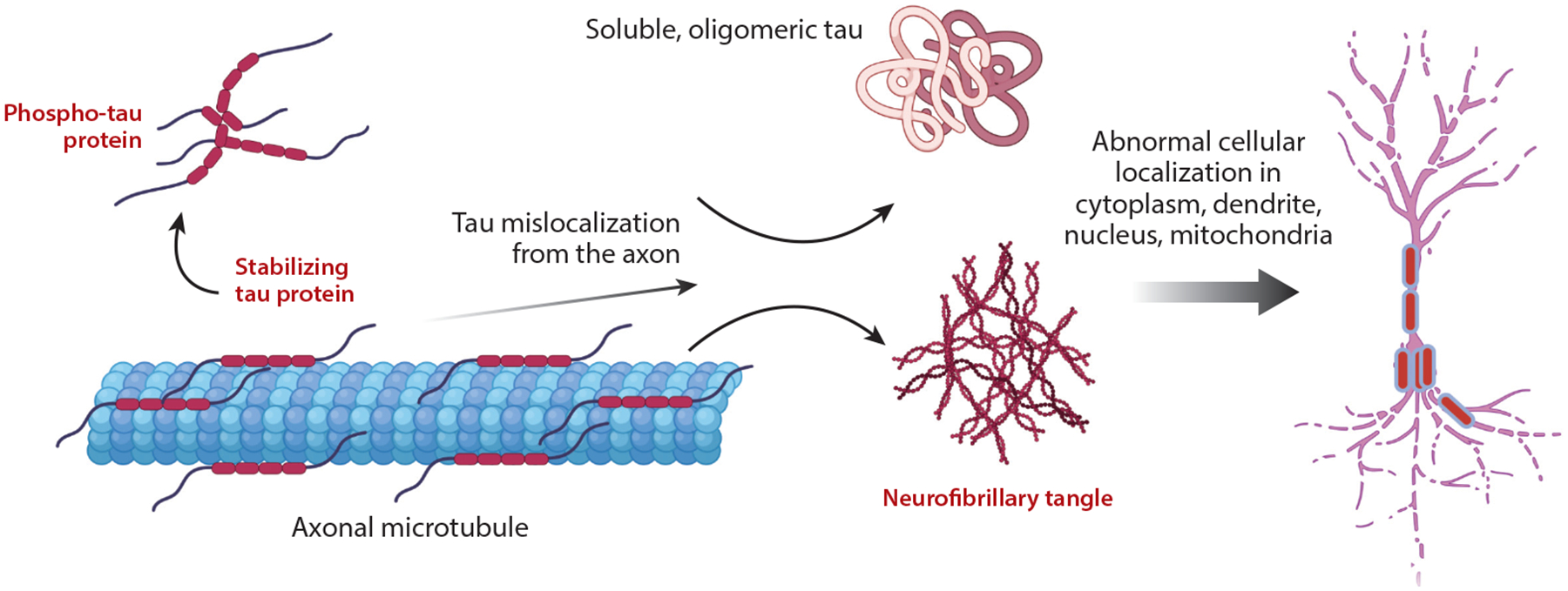
Tau Pathology and Mechanisms
Project Overview
Our lab focuses on understanding tau kinetics, including fibrillar and oligomeric forms, to elucidate their replication and spreading rates in the brain, key to Alzheimer’s progression. Using chemical kinetics, proteomics, and machine learning, we quantify tau aggregation and investigate why therapies effective in mouse models often fail in humans. We study tau-related neuronal resilience, GWAS-validated tauopathy genes (e.g., JADE1), and tau’s impact on neuronal firing critical for memory. We also examine pathogenic tau’s role in activating transposable elements and mislocalization of nuclear pore proteins like NUP98.
Learn More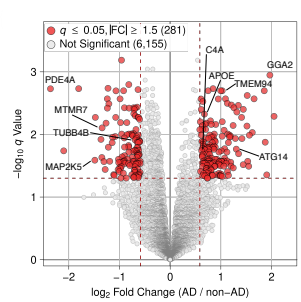
Transcriptomics and Neurodegeneration
Project Overview
We investigate the genomic and transcriptomic alterations underlying neurodegenerative diseases. Our findings reveal that neuronal DNA damage accumulates progressively in Alzheimer’s disease, driven by pathogenic mechanisms. We analyze region-specific endothelial cell heterogeneity and transcriptomic responses to amyloid-β plaques, tau tangles, and APOE variants. Additionally, we explore astrocyte transcriptomic changes over disease progression and identify therapeutic targets such as STMN2 and UNC13A in TDP-43 proteinopathies.
Publications
- Das, S. et al. (2023). “Distinct transcriptomic responses to Aβ plaques, neurofibrillary tangles, and APOE in Alzheimer’s disease.” Alzheimer’s & Dementia 20(1), 74-90.
- Wachter, A. et al. (2024). “Landscape of brain myeloid cell transcriptome along the spatiotemporal progression of Alzheimer’s disease reveals distinct sequential responses to Aβ and tau.” Acta Neuropathologica 147(1), 65.
- Collins, M. et al. (2025). “Ubiquitin-Proteasome System Dysregulation in Alzheimer’s Disease Impacts Protein Abundance.” [Preprint]
Learn More
Featured Publications
Alzheimer disease-associated tau post-translational modification mimics impact tau propagation and uptake
John R. Dickson, Robert G. R. Sobolewski, Analise R. Fernandes, Joanna M. Cooper, Zhanyun Fan, Mirra Chung, Cameron Donahue, Derek H. Oakley, Dudley K. Strickland, Bradley T. Hyman (2025)
Journal of Neuropathology & Experimental Neurology
PDFAlzheimer proteopathic tau seeds are biochemically a forme fruste of mature paired helical filaments
Mukesh Kumar, Noé Quittot, Simon Dujardin, Christoph N. Schlaffner, Arthur Viode, Anne Wiedmer, Pieter Beerepoot, Joshua E. Chun, Calina Glynn, Analiese R. Fernandes, Cameron Donahue, Judith A. Steen, Bradley T. Hyman (2024)
Brain
PDFAPOE2 gene therapy reduces amyloid deposition and improves markers of neuroinflammation and neurodegeneration in a mouse model of Alzheimer disease
Rosemary J. Jackson, Megan S. Keiser, Jonah C. Meltzer, Dustin P. Fykstra, Steven E. Dierksmeier, Soroush Hajizadeh, Johannes Kreuzer, Robert Morris, Alexandra Melloni, Tsuneo Nakajima, Luis Tecedor, Paul T. Ranum, Ellie Carrell, YongHong Chen, Maryam A. Nishtar, David M. Holtzman, Wilhelm Haas, Beverly L. Davidson, Bradley T. Hyman (2024)
Molecular Therapy
PDFOur Team

Dr. Jane Smith
Principal Investigator
Robert (Sobie) Sobolewski
Alumni
XXX
Research Technician
Dr. Michael Johnson
Postdoctoral Researcher